Articles by Cody Barnett, MPH, MRA Senior Director of Communications & Patient Engagement
Keeping You Connected to the Melanoma Community
By Cody Barnett, MPH, MRA Senior Director of Communications & Patient Engagement | 18 February 2020 In Allies & Partnerships
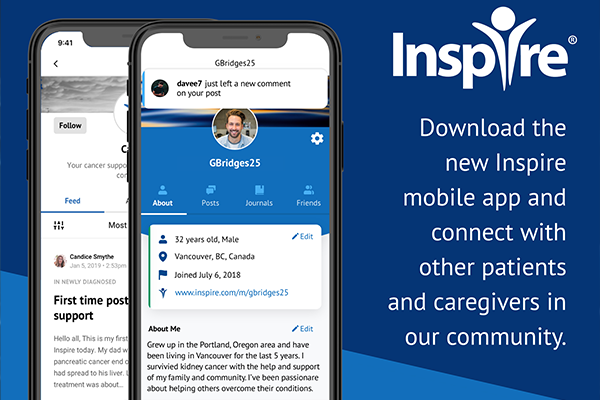
With the launch of the brand-new Inspire App, the Melanoma > Exchange community is getting even better. Available for iPhone and iPad – the app was designed from the ground up to make connecting and sharing even easier and as mobile as you are.
Stage 4 Melanoma, a Vaccine Clinical Trial, and the Power of Family & Faith
By Cody Barnett, MPH, MRA Senior Director of Communications & Patient Engagement | 7 February 2020 In Melanoma Stories, Science, Treatment
“Clinical trials allow you to take advantage of the latest research and experiences from doctors and patients who’re blazing the trails. Clinical trials are also a way for you to help the next person who is going to have to go through this. If you have the opportunity to be part of something that helps others, why wouldn’t you at least try it?”
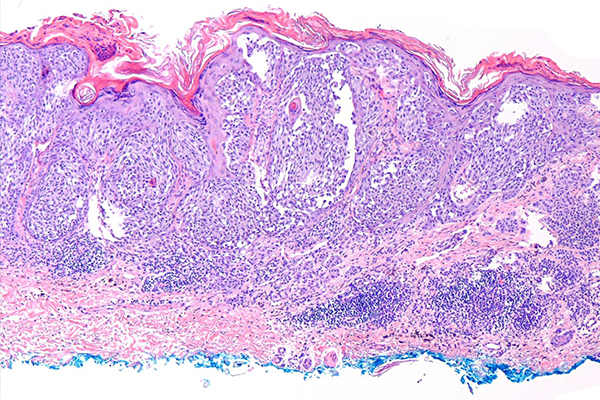
Brown Skin Too: If You Have Skin, You Can Get Skin Cancer
By Cody Barnett, MPH, MRA Senior Director of Communications & Patient Engagement | 6 December 2019 In Melanoma Stories, Prevention
While pervasive, the myth that people of color don’t need to worry about skin cancer, is entirely untrue. And while the rates of skin cancer among people of color are lower than rates for people with lighter skin, low risk doesn’t mean no risk.
Mutations and Melanoma
By Cody Barnett, MPH, MRA Senior Director of Communications & Patient Engagement | 8 November 2019 In Science
Cancer is caused by mutations in our DNA that allow cells to grow uncontrollably – and eventually invade surrounding tissue. Melanoma is a specific type of cancer that is formed in pigment-containing cells, known as melanocytes, which are found primarily in the skin but also in places like the eye and on mucous membranes. Thanks to advancements in research, the same mutations that cause cancer are proving helpful in treating it.
Bob Enrolls in Clinical Trial Testing NeoAdjuvant Therapy for Melanoma
By Cody Barnett, MPH, MRA Senior Director of Communications & Patient Engagement | 2 November 2019 In Melanoma Stories, Science, Treatment
After being diagnosed with Stage 3 melanoma, Bob chose to enroll in a clinical trial at Georgetown University, comparing the effectiveness of treating melanoma with pembrolizumab before or after surgery, what doctors call neo-adjuvant and adjuvant therapy respectively.
SPORE Grants: Putting Collaboration Front & Center
By Cody Barnett, MPH, MRA Senior Director of Communications & Patient Engagement | 24 September 2019 In Allies & Partnerships, Science, Treatment
In medicine, sometimes the biggest and most game-changing advancements come from a single person – who through tireless work – arrives at a true ‘eureka’ moment. This ‘go it alone’ concept is not only reinforced through television and movies, it’s also bolstered in the way the medical community makes research...
Living Life With Melanoma
By Cody Barnett, MPH, MRA Senior Director of Communications & Patient Engagement | 13 September 2019 In Melanoma Stories
Ken Billett’s life-long journey with melanoma began in 1995 when he took a leap of faith and had a spot checked out on his shoulder at a health expo. He knew that he had had more than his fair share of sunburns growing up in Florida during the 60’s and...
One Day at a Time: Dylan Overcomes Depression & Melanoma
By Cody Barnett, MPH, MRA Senior Director of Communications & Patient Engagement | 12 August 2019 In Melanoma Stories
Melanoma taught Dylan to stay in the moment and to tackle things one day at a time. There were so many things that were outside of Dylan's control, so he decided to focus on the things that he could do something about.
Acral Melanoma & Adjuvant Therapy – One Patient’s Decision
By Cody Barnett, MPH, MRA Senior Director of Communications & Patient Engagement | 23 July 2019 In Melanoma Stories, Treatment
When David was diagnosed with Stage 3 acral melanoma he faced a major decision. The surgery to remove it was successful - but should he move forward with adjuvant therapy to reduce the risk of it returning? Learn more about the benefits - and risks - of this approach and read what David decided.
A Better Combination?
By Cody Barnett, MPH, MRA Senior Director of Communications & Patient Engagement | 19 July 2019 In Science
The FDA's 2015 approval of the first ever checkpoint immunotherapy combination of ipilimumab + nivolumab was considered a major breakthrough. Since then, research has shown that the combo yields slightly better results at the cost of increased side effects. Now, a new study asks if we can optimize the dose to produce the same effects with fewer side effects.
About the Author
Cody Barnett, MPH, MRA Senior Director of Communications & Patient Engagement
Cody Barnett, MPH serves as the Director of Communications & Patient Engagement at the Melanoma Research Alliance (MRA). In this role, he works to develop and cultivate strategies, messages, and stories that propel MRA and its mission forward. In addition, he develops patient-friendly articles, tools, and resources that demystify new research advances and make them actionable. Cody is responsible for media relations, web and social marketing, publications and materials development, and organizational branding. He is committed to using the power of storytelling and patient-centered thinking to advance melanoma research and improve patient outcomes.
Before joining the Melanoma Research Alliance, Cody served as Director of Communications at AIDS United, where he directed all internal, public, member, and online communications for the national HIV-focused organization. Previous to this, he managed digital communications for the Robert Wood Johnson Foundation’s signature initiative, Aligning Forces for Quality (AF4Q).
Cody earned his Master of Public Health (MPH), concentrating in health policy, from The George Washington University Milken Institute School of Public Health in 2013 and a Bachelor of Arts in political science from York College of Pennsylvania.