.png)
One major focus of cancer research is genetics - the study of changes in DNA that can drive the development of cancer. These genetic alterations may be inherited (helping to explain why cancer can run in families) or acquired over an individual’s lifetime. One way that physicians may diagnose and characterize cancer in their patients is by using tests that look at changes in DNA-such as gene mutations-that occur in healthy cells (inherited mutations) and cancer cells (acquired mutations). An understanding of these genetic alterations is important for informing cancer risk, diagnosis, prognosis, and treatment.
Compared to cutaneous (skin) melanoma, much less is known about the genetic risk factors (inherited mutations) or genetic tumor biomarkers (acquired mutations) present in patients with rare melanoma subtypes such as acral and mucosal melanoma. To help advance research in this area, the Melanoma Research Alliance (MRA) sponsored the first direct-to-patient registry called RARE to collect information on the patient perspective and disease journey from participants with cutaneous, acral, or mucosal melanoma. Using patient-reported data from the RARE Registry, we have examined for the first time the use of genetic and biomarker testing in the medical journeys of participants diagnosed with cutaneous, acral, or mucosal melanoma.
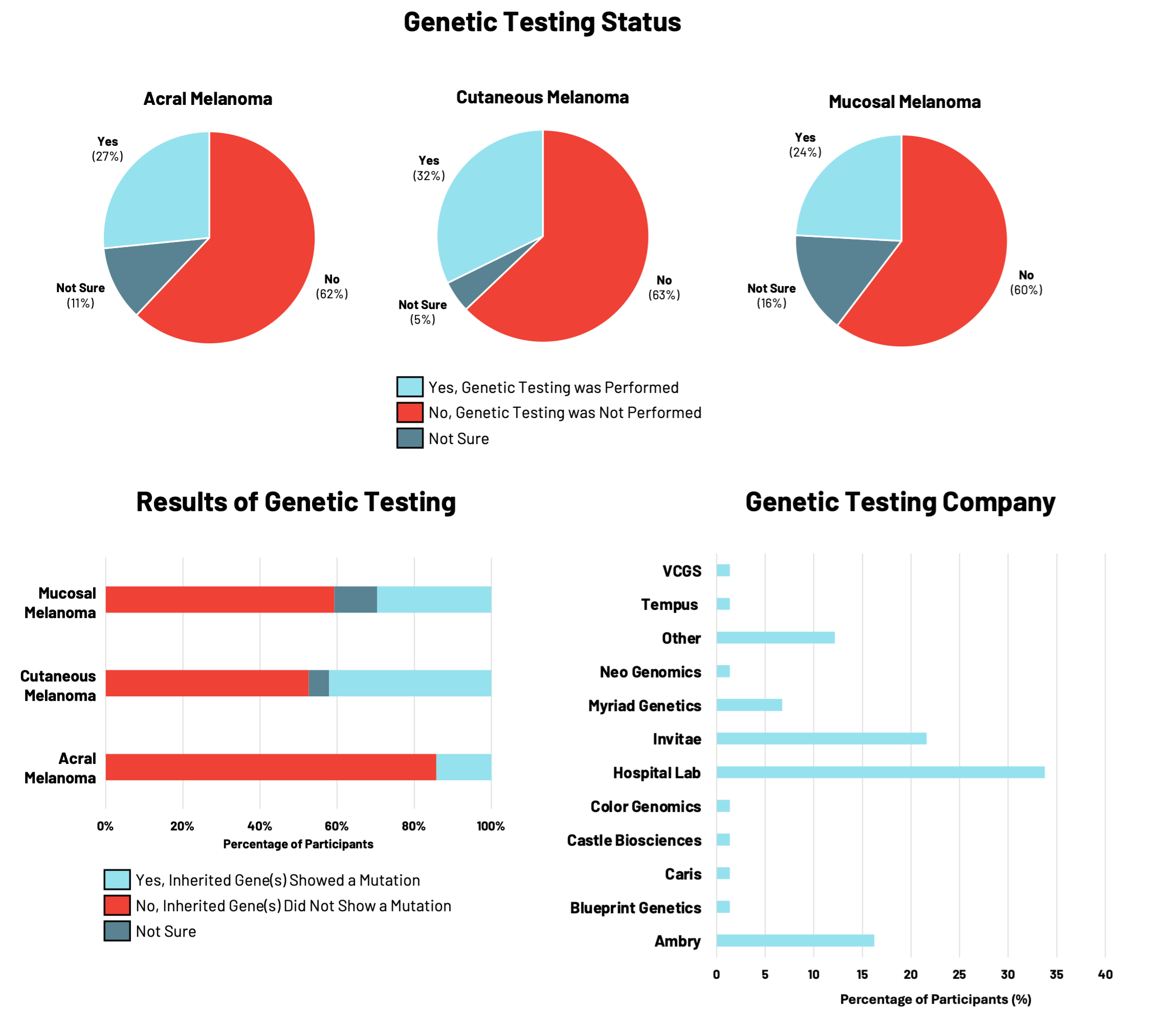
Genetic testing is used to identify inherited changes in genes of healthy cells that may increase an individual’s risk for developing specific types of cancer. For a subset of melanomas, inherited changes in genes may increase an individual’s lifetime risk of developing the disease.
Given the importance of genetic testing in assessing melanoma risk and the RARE Registry’s goal of advancing knowledge about hereditary melanoma across all subtypes, we looked at the use of genetic testing by participants enrolled in RARE (Figure 1).
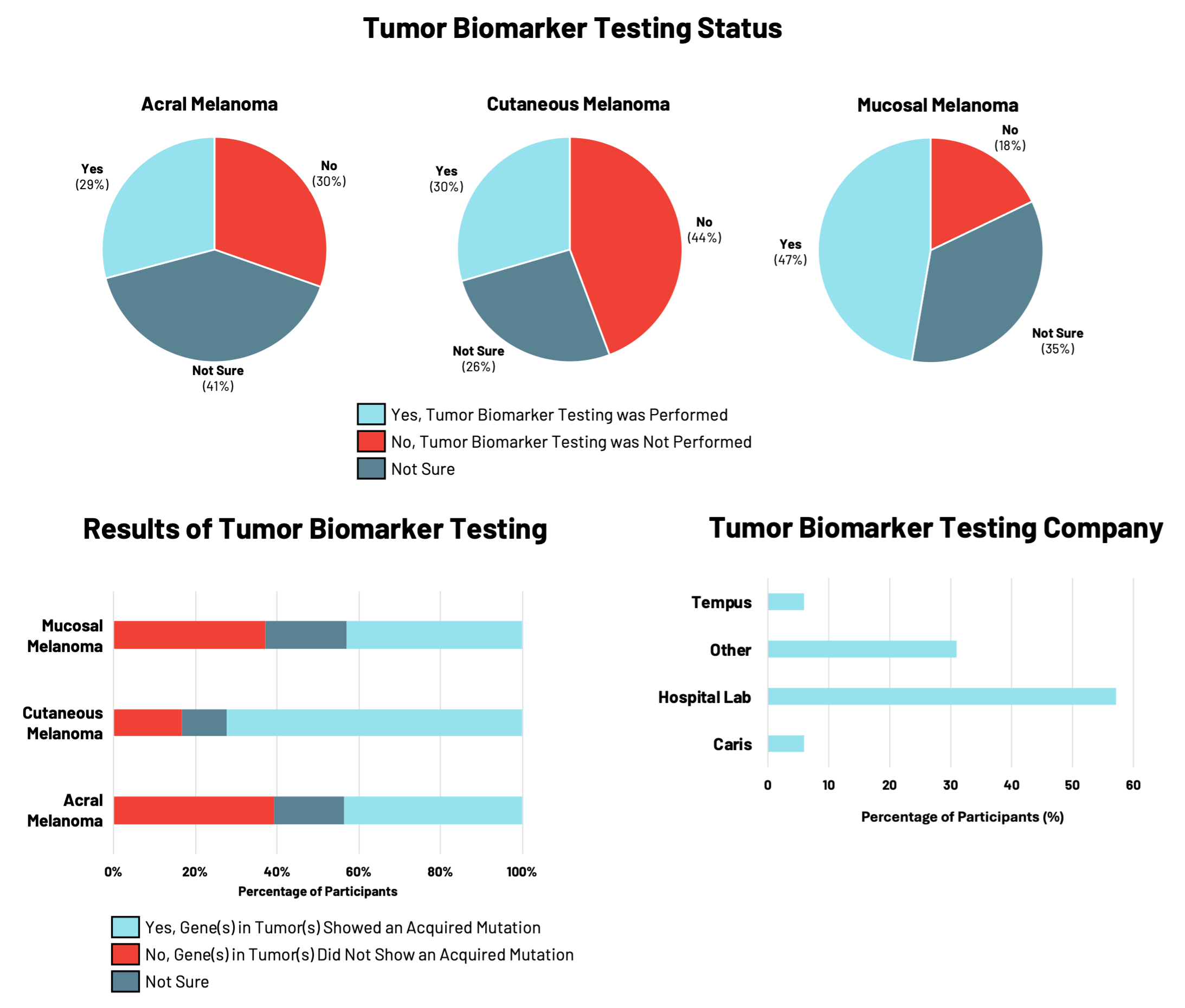
One type of tumor biomarker testing looks at the genetic changes in DNA or genes present in cancer cells(acquired mutations) and helps physicians to understand more about a patient’s specific cancer, including prognosis and personalized treatments. Studies of genetic tumor biomarkers in acral and mucosal melanomas are emerging: these rare subtypes have been found to harbor fewer acquired mutations and have distinct mutational profiles compared to cutaneous melanomas.
RARE Registry participants were asked whether their melanoma tumors had biomarker testing performed that specifically looked for acquired (not inherited) gene mutations in the DNA of melanoma tumor cells. For patients with melanoma, understanding the genetic changes acquired by the tumor cells is important for making treatment decisions.
In the RARE registry, participants with mucosal melanoma were the most likely to have had tumor biomarker testing performed (47%), compared to those with acral or cutaneous melanomas (<30%) (Figure 2). Of these participants, tumor biomarkers were identified in 72%of individuals with cutaneous melanoma, compared to approximately 43% of individuals with either acral or mucosal melanoma. Differences in the specific tumor biomarkers found by testing were observed across all three melanoma subtypes.Hospital-affiliated labs were most commonly used for tumor biomarker testing, followed by clinical diagnostic companies like Tempus, Caris, and others.
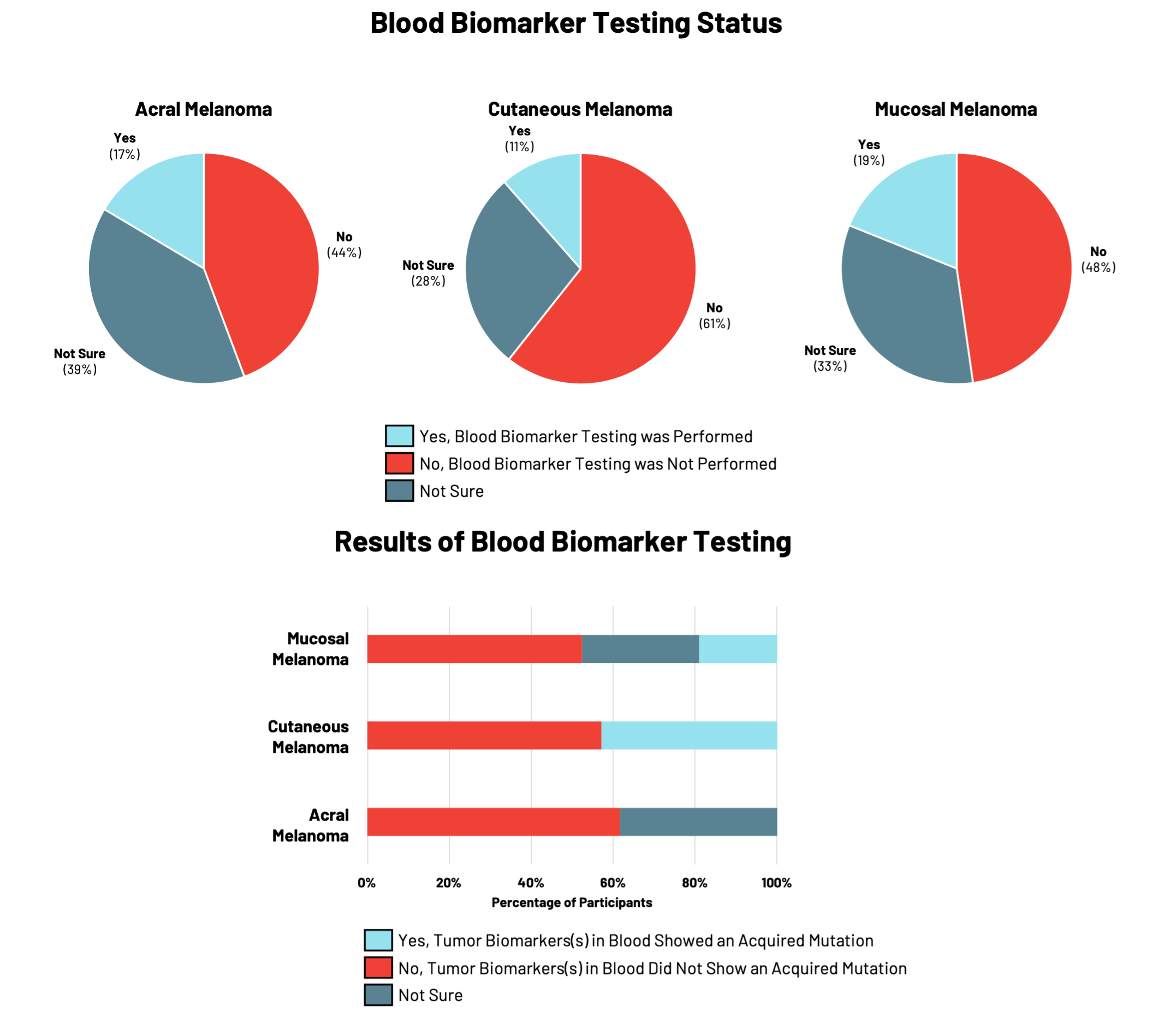
Blood biomarker testing measures specific molecules in blood samples (such as DNA) to monitor the progression of an individual’s cancer and responses to particular treatments. This is a newer type of testing that is starting to be used by more physicians to monitor how well a treatment is working in patients with cancer.
For RARE registry participants, blood biomarker testing was the least common type of testing performed relative to genetic and tumor biomarker testing with 11% to 19% of participants having had testing performed (Figure 3). Participants with cutaneous or mucosal melanoma reported the presence of tumor biomarkers showing acquired mutations in cancer risk genes.
Collectively, this initial look at RARE Registry data indicates that this patient-driven platform is valuable for capturing meaningful data across melanoma subtypes, and can enable collaborative efforts between patients and researchers to advance knowledge and use of genetic and biomarker testing in both common and rare melanoma subtypes.
These findings represent the first in a series of insights emerging from MRA’s RARE Registry. Interested in participating in the RARE Registry? Learn more about how to become involved in this initiative by visiting raremelanoma.org.
Visit MRA’s dedicated resource pages to learn more about genetic and biomarker testing. If you are interested in genetic and biomarker testing, speak to your healthcare provider about potential options. It is important to ask what information these types of testing can provide, the limitations of such tests, and how testing can impact your current and future care.