.png)
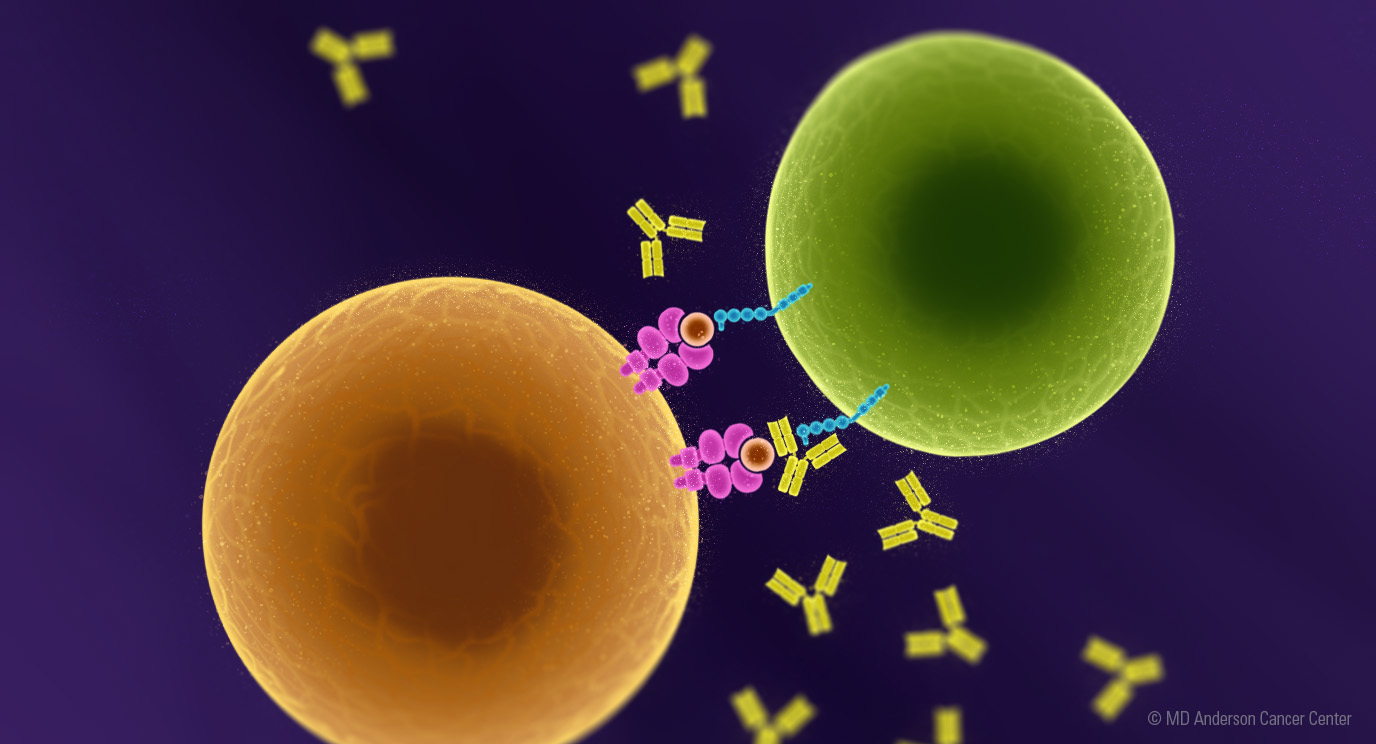
Immune cells have molecules called checkpoints, which put the brakes on the immune system’s ability to find and kill cancer cells. Immunotherapy treatments called immune checkpoint inhibitors (ICIs) work by blocking these molecules, helping to release the brakes off the immune cells so that they can find and kill cancer cells.
Today, most FDA-approved ICIs for advanced melanoma target the checkpoints PD-1 and CTLA-4:
These therapies have revolutionized the melanoma treatment landscape - but they don't work for everyone. Many patients either don’t initially respond or eventually develop resistance.
To learn more about these ICI treatments, including those currently in clinical trials, we talked to Dr. Hussein Tawbi, Professor and Deputy Chair of the Department of Melanoma Medical Oncology at MD Anderson Cancer Center in Houston, Texas.
LAG-3 stands for lymphocyte-activation gene 3 and it is the third immune checkpoint to be clinically validated in melanoma, after PD-1 and CTLA-4. It’s found on the surface of T cells - your immune system’s frontline fighters.
Both LAG-3 and PD-1 can put the brakes on immune responses to cancer cells.1 Dr. Tawbi explained that LAG-3 and PD-1 contribute to a phenomenon called “T cell exhaustion.” This means that over time, T cells that have learned to recognize tumor cells are blocked from killing them. LAG-3 and PD-1 are markers of T cell exhaustion. Researchers are learning that blocking both checkpoint molecules works better than blocking only one.2 Blocking both at the same time more completely reverses the T cell exhaustion and restores the ability of theT cells to kill melanoma cells.
Nivolumab, an ICI that blocks PD-1, and relatlimab, which blocks LAG-3, were tested in a phase 2/3 clinical trial. Results showed that 43% of patients who received the nivolumab + relatlimab combination responded to the treatment.3 The combination of nivolumab + relatlimab (Opdualag®) was FDA approved for advanced melanoma in 2022.
To more broadly address patients with melanoma, such as those with melanomas that can be removed with surgery (called resectable) as well as melanomas that cannot be surgically removed (called unresectable), biotechnology company Regeneron is testing the combination of a novel LAG-3-blocking drug called fianlimab and a PD-1-blocking drug called cemiplimab (Libtayo®) that is approved for use in other cancers. Four clinical trials in phase 2 or 3 are being conducted to test this combination in patients with different stages of melanoma and at different times during their treatment journey.
New treatment options for melanoma are first tested in clinical trials, which are research studies that involve human volunteers with melanoma. Researchers use clinical trials to test new treatments and/or a new combination of existing treatments. The treatments under study in clinical trials have already undergone rigorous testing to minimize any safety risks for patients. The goal of a clinical trial is to determine whether a new therapy or combination improves patient outcomes compared to existing treatments. Researchers also use the information from clinical trials to determine dosage amounts and treatment schedules, and to evaluate side effects.
Clinical trials have the potential to offer patients access to treatment approaches that may be more promising and potentially more beneficial than those currently approved by the FDA. In addition, clinical trials drive our understanding of melanoma forward, improving future treatment options for all patients. Patients are encouraged to consider all their treatment options, including discussing with their physician the potential of enrolling in a clinical trial.
Two of Regeneron’s four fianlimab + cemiplimab trials are investigating the combination in patients with resectable melanoma. A phase II trial (NCT06190951) is currently recruiting and testing if the combination of fianlimab + cemiplimab works better than cemiplimab alone when given before surgery (called neoadjuvant therapy) as well as after surgery if needed in patients with melanoma at high risk of recurring. Also, the phase 3 Harmony Adjuvant trial (NCT05608291), while not actively recruiting participants, is comparing the standard of care treatment, pembrolizumab (Keytruda®), with the combination of fianlimab + cemiplimab when given after surgery (called adjuvant therapy) for preventing or delaying melanoma from coming back in patients who are at high risk for recurrence of the disease.
The other two fianlimab + cemiplimab trials are for those with advanced and metastatic melanoma that cannot be completely removed with surgery. The phase 3 Harmony Melanoma trial (NCT05352672) is assessing how well the combination of fianlimab + cemiplimab works compared to pembrolizumab alone (approved for the treatment of advanced melanoma) in patients with previously untreated advanced melanoma. The phase 3 Harmony Head to Head trial (NCT06246916) is testing the efficacy of fianlimab + cemiplimab compared to the approved combination of nivolumab + relatlimab in patients with previously untreated advanced melanoma. The Harmony Head to Head trial is currently enrolling participants but the Harmony Melanoma trial has just closed enrollment.
Regeneron’s large melanoma clinical development program is supported by data from a phase I clinical trial (NCT03005782) that examined the combination of fianlimab + cemiplimab. Of the 98 patients with advanced melanoma who had never received treatment with anti-PD-1 therapy, 57% of patients responded, and 25% had a complete response, meaning their cancer disappeared. Among those who had received a prior anti-PD-1 drug (for at least 6 months), 46% of patients responded, and 31% had a complete response.4 Safety of the fianlimab + cemiplimab combination was similar to that of other single-agent anti-PD-1 therapies. A higher frequency of adrenal insufficiency was noted with this combination than with other types of immunotherapy.
When considering enrolling in a trial testing the combination of fianlimab + cemiplimab, Dr. Tawbi explained that a challenge is that adrenal insufficiency can be subtle and difficult to recognize. “Patients and doctors should be aware of this possible side effect and test for it early during treatment,” he explained. Patients should also be aware that the fianlimab + cemiplimab combination is given more frequently - every 3 weeks - rather than every 4 weeks for the FDA-approved nivolumab + relatlimab.
An advantage that Dr. Tawbi is excited about is that because the combination of fianlimab + cemiplimab is fairly safe, other drugs could be added. “This possibility could improve outcomes for patients even more,” he said.
Important questions remain to be answered about the role of LAG-3 + PD-1 in different situations such as:

Biomarkers that can predict who will or will not respond to blockade of LAG-3 and PD-1 are also needed. Other questions remain around dosing, as each LAG-3 antibody is a different molecule with different doses. Researchers continue to look for the dose of each drug that works the best with the fewest side effects.
Collectively, the goal of the four trials testing the combination of fianlimab + cemiplimab is to help a broad group of people with melanoma. The trials are enrolling patients with melanoma that is resectable or unresectable. These studies are comparing the novel combination with approved treatments.
Dr. Tawbi believes that fianlimab + cemiplimab is a “compelling combination”, and he is very excited about the possibility of its broad use. He would like patients to know that “a treatment does not have to be toxic to work”. This combination may be useful early after diagnosis of melanoma, allowing more toxic treatments to be reserved for a later time and only if necessary.